

Silver carbonate reacts with ammonia to give the explosive silver fulminate.

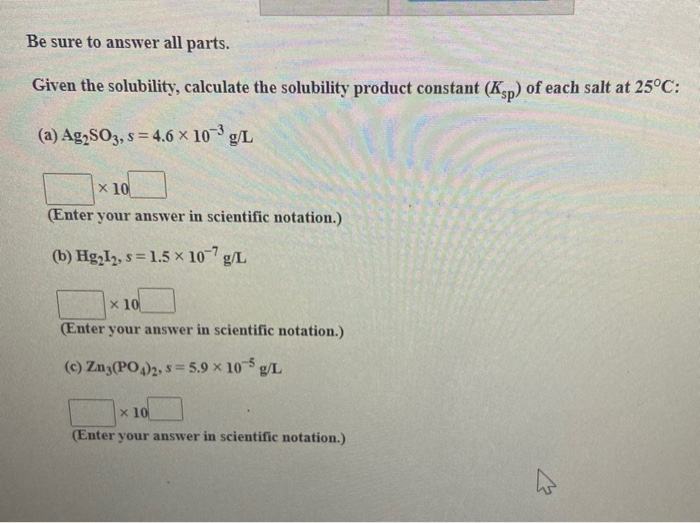
Ag 2 CO 3 + 2 HNO 3 2 AgNO 3 + H 2 O + CO 2. How do you calculate the solubility of silver sulfate in each of the following: a). Like all other carbonates, silver carbonate will react with acids to give their respective silver salts and give off carbon dioxide. Solubility products are determined experimentally by directly measuring either the concentration of one of the component ions or the solubility of the compound in a given amount of water. The Ksp for silver sulfate (Ag2SO4) is 1.210-5. \): Solubility Products for Selected Ionic Substances at 25☌ Solid Chemistry: The Central Science 14th Edition ISBN: 9780134414232 (6 more) Bruce Edward Bursten, Catherine J.


 0 kommentar(er)
0 kommentar(er)
